
hydrogen absortion and emission spectra showing the
Emission spectrum of hydrogen

The bottom spectrum is the absorption spectrum of hydrogen.

The emission and absortion spectrum of hydrogen in the visible

corresponding to the atomic emission spectrum of the element Hydrogen.

Atomic line spectrum for hydrogen, mercury and helium.

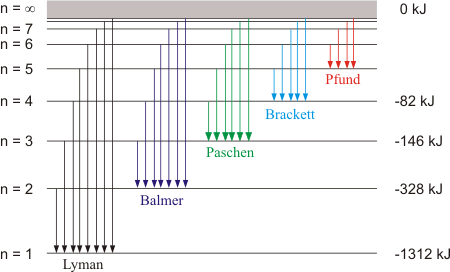
Emission Spectrum of Hydrogen

Visible Light Spectrum and Hydrogen Emission/Absorption Spectra

So Hydrogen's spectrum in the visible region looks like this.

Example of emission spectrum: hydrogen; (from bluegiant.phys.ksu.edu)

hydrogen line spectrum emission and absorption

Emission Spectrum Of Sample Elements - The emission spectra of some
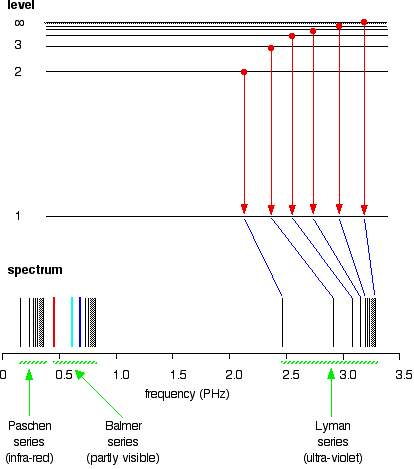
atomic hydrogen emission spectrum
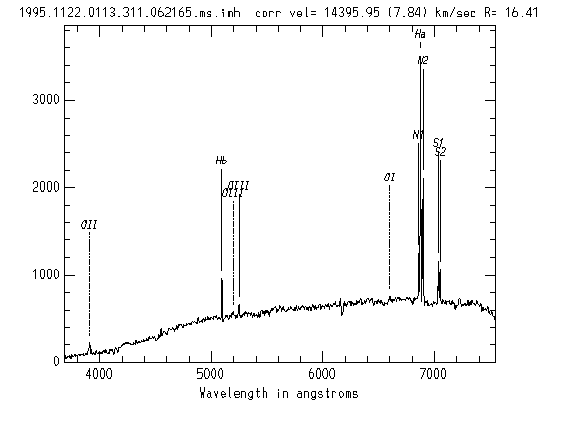
Spectrum with Labelled Emission Lines. xcsao summary page with labelled
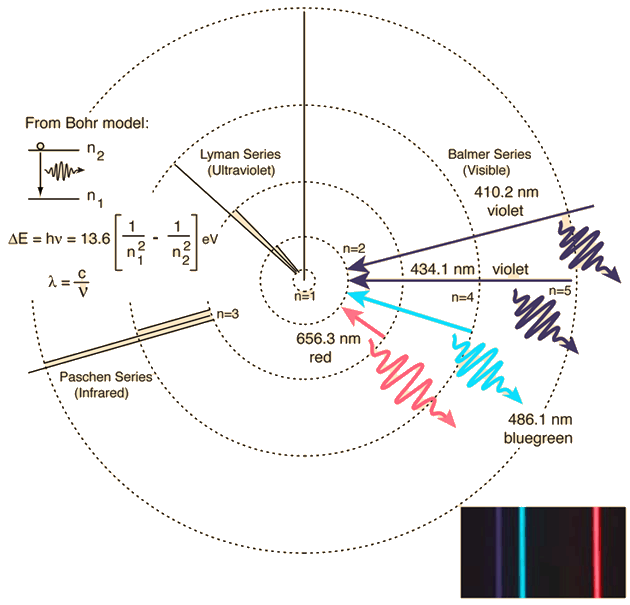
This spectrum was produced by exciting a glass tube of hydrogen

Here are some simple "emission spectra" that are emitted when material is

Emission Spectra

Visible Light Spectrum and Hydrogen Emission/Absorption Spectra

The image below shows examples of a continuous spectrum and an emission line

Emission lines for different materials. Hydrogen:

